Optimal Clinical Peer Review Case Volume
Question: What is the optimal case volume for peer review?
Short Answer: Between 1-5% of hospital admissions
Detail:
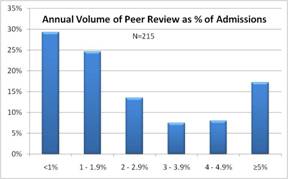
In our national study of peer review practices, we asked for estimates of peer review case volume in relation to overall hospital volume. (1) Among 46%, case volume exceeds 2% of hospital volume. More importantly, we found that case volume was a factor contributing to perceived quality impact of the peer review program.
A benefit was apparent above 1% of hospital inpatient volume. This may seem surprising in relation to QI principles, which seek to minimize the cost of inspection by designing quality into the process.
The explanation may be that clinical care is largely a poorly controlled process. The Harvard Medical Practice Study (HMPS) and similar investigations have shown that adverse event rates typically exceed 3%, with physician management issues contributing to about a third of these. (2) More recent studies using the robust IHI Trigger Tool methodology show much higher rates. Among Medicare patients, preventable adverse event rates are around 7%. (3) Thus, programs with review volume lower than 1% could not identify most individual improvement opportunities.
Also, if case volume is low, it will be difficult to “shift the curve” via meaningful clinical performance feedback to a significant proportion of the active medical staff. Clinical performance measurement and timely feedback are essential components of the QI Model for peer review.
While the optimal case volume for the QI Model needs to be evaluated by further study, the related question of how to best identify cases for peer review still needs to be answered. In the OIG study, nurse screening of medical records was combined with coding review to exclude cases with Present on Admission indicators for the targeted diagnoses and with claim history review to include those with a prior hospitalization within 30 days. Even so, 50% of the original random sample of cases required second stage review by physicians to identify those with preventable adverse events. Such a process is too inefficient for general use.
- Edwards MT, Benjamin EM. The process of peer review in US hospitals. J Clin Outcomes Manage 2009(Oct);16(10):461-467.
- Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients: results of the Harvard Medical Practice Study I & II. NEJM 1991;324(6):370-384.
- Office of Inspector General. Adverse Events in Hospitals: National Incidence among Medicare Beneficiaries. OEI-06-09-00090. November 2010. http://oig.hhs.gov/oei/reports/oei-06-09-00090.pdf